Obligations of EU Exporters
 Notification Obligations
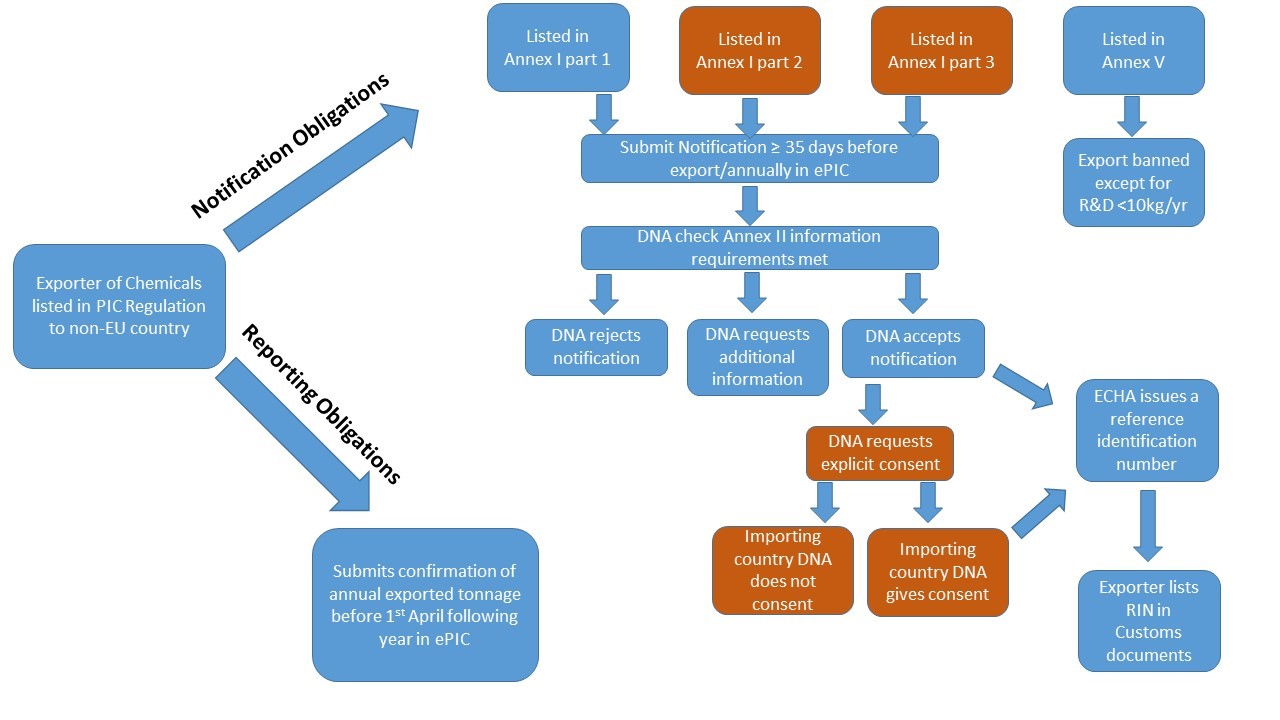
Notification Obligations
Export of Annex I chemicals on their own or in mixtures at or above the relevant specific or generic concentration limits for CLP classification (Annex I or VI), requires an active Reference Identification Number (RIN) to be documented in Box 44 of the Single Administration Document (SAD), for presentation to Customs Authorities. To obtain a RIN, the intended export of these chemicals must be notified in ePIC at least 35 days prior to the date of proposed export and annually thereafter. The information required for submission of an export notification is listed in Annex II to the Regulation and in ECHA’s Guidance for Industry. Since the withdrawal of the UK from the EU on 1 January 2021, any export of chemicals listed in Annex I of the PIC Regulation from Ireland to Great Britain (UK (GB)), will require prior notification to the Irish designated national authority.
The exporting country’s DNA will determine whether to accept or reject the notification in ePIC, or whether to request clarification or additional information from the exporter. Notifications may be rejected if the same exporter has already submitted a notification for the same substance or mixture with proposed export to the same destination country. Additional information may be requested such as:
- Information on Labelling and Packaging, to ensure that at least the same standards as that required within the EU is met and, if practicable in the language of the importing country
- Information supplied in a safety data sheet (SDS) is in accordance with Annex II of the REACH Regulation (EC) No. 1907/2006.
- The SDS should be provided in English and if different, in an official or principal language of the importing country (see language requirements for importing countries in Appendix 4 of the PIC Guidance document)
- The non-EU importer’s contact name, full address and telephone number are provided. Please note that a P.O. box cannot be accepted
- The expiry date and production date of the chemical are indicated on the label, and is not less than six months before its expiry date
For Annex I part 2 & 3 chemicals, explicit consent from the importing country’s designated national authority (DNA) is requested by the exporting country’s DNA (HSA or DAFM in Ireland) and recorded in ePIC. An active RIN is issued only where the importing country’s DNA has provided explicit consent for the export. Where the importing country does not respond, a waiver may be applied under certain specific circumstances, please see ECHA’s waiver webpage for more details.
Notification Obligations for Annex I substances in articles
In the PIC Regulation, articles are defined as a finished product containing or including a chemical, the use of which has been banned or severely restricted by Union legislation in that particular product. Articles are subject to export notification if they contain an Annex I chemical on its own or in a mixture at or above the relevant specific or generic concentration limits for CLP classification (Annex I or VI). An example of an article requiring export notification is mercury in measuring devices subject to REACH Restriction. The concentration of the listed chemical within the article does not matter as an export notification is required irrespective of this concentration.
Certain chemicals and articles listed in Annex V to the Regulation are banned for export.
Notification Obligations for Exports used in Research & Development
The Export Import Regulation does not apply to Annex I or V chemicals exported for the purposes of research or analysis in quantities that are unlikely to affect human health or the environment and that in any event do not exceed 10 kg from each exporter to each importing country per calendar year. However at least 35 days prior to planned export, exporters of such chemicals need to obtain a special RIN from their DNA using ePIC, and include this code in box 44 of the Single Administrative Documents (SAD). Please see ECHA’s Special RIN Requests document for further details.
Reporting Obligations
By 30th March each year, EU exporters of any chemical listed in Annex I of the Export Import Regulation shall inform their DNA of the actual quantity of:
(a) substances listed in Annex I;
(b) mixtures containing such substances in a concentration that triggers labelling obligations under CLP irrespective of the presence of any other substances; or
(c) articles containing substances listed in Part 2 or 3 of Annex I in unreacted form or mixtures containing such substances in a concentration that triggers labelling obligations under CLP irrespective of the presence of any other substances;
exported in the previous calendar year using ePIC (Article 10 report) regardless of the intended use of the chemical. Please note that Article 10 reports are not required for Annex I substances, on their own or in mixtures, exported for R&D use below 10kg per calendar year. Please see the ePIC Industry User Manual for advice on completing this report.
Import Responses
A link to the recent PIC circular published in June 2022 is available here: http://www.pic.aspx
Import responses are the decisions provided by non-EU/EEA countries on whether or not they will allow the import of certain chemicals subject to the PIC Regulation. Since the 1st of January 2021, this also includes Great Britain. The obligations of parties in relation to the import of chemicals listed in Annex III are set out in Article 10 of the Convention. Each party provides a response related to the future import of a certain chemical. This may be a final decision or an interim response. New import responses submitted by parties are published in the PIC circular every six months (in June and December).
Further Information
To assist you in determining your role under our chemical legislation, please visit our 'Role Finder Tool'.